Around the Office
-
2023 Keystone Holiday Celebrations

Keystone has some annual holiday celebrations during the month of December that give our staff and families an opportunity to gather together, enjoy some delicious food, reflect on what we've accomplished over the last year, and discuss what might be possible in the next. This year, our Holiday Dinner and our office potluck / white elephant gift exchange happened on consecutive days.
On the evening of Tuesday, December 19, we gathered around a long table in a private room at Cafe Luna in downtown Raleigh for an evening of wonderful Italian food. The conversation was lively and everyone enjoyed the opportunity to catch up on the lives of our coworkers outside of the office.
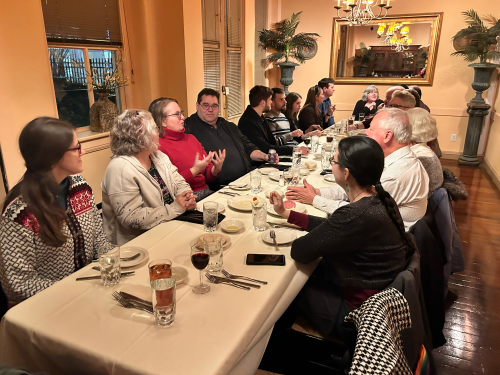
Just after noon on Wednesday, December 20, we gathered in our project area for our annual office potluck. We are always impressed by the variety of choices once everyone's contribution is added to the buffet spread. Our staff even thoughtfully create dishes taking into account known food allergies and sensitivities, so there's something to appeal to everyone and no one goes hungry!

After our potluck lunch, we do a white elephant / dirty Santa gift exchange. Our rules are: only one steal per gift per round, and everything's in play until the person with the last number's round. Does your organization do a white elephant exchange? If so, what are your rules?

I hope you enjoyed a brief look at some of this year's Keystone holiday celebrations. Hopefully, your year end also included festive gatherings such as ours, or at least some good rest and relaxation.
Happy Holidays and Happy New Year from all of us!
-
A look back: 2022 in Review

Another year is in the books, and we’re stoked to be officially in a conference year! But, before we barrel ahead, let’s take a look back at 2022.
Around the Office
The Keystone offices were a little less quiet this year as some of the staff have transitioned back to working from the office on a regular basis. However, there have been even bigger transitions as we've seen some staffing changes.
Longtime developer Brian White and customer support specialist John Owen retired, but new faces George and Katharina have joined the family in their stead.
Events & Training
This past summer, we held our 2022 KLAS Mini-Conference to help fill the gap between conference years. Thank you to everyone who joined us and helped make it a successful event! We hope to see you all again either in-person or online for the 2023 Users Conference!
We also held the first online IRC Administrator's Training! Thanks as well to our first round of IRC Admins, and we hope everything you learned has been serving you well.
Last but not least, we want to highlight the Onboarding New KLAS Users webinar. If you've had staffing changes of your own, or expect to bring on some new staff in the new year, make sure to check it out!
KLAS Development
Finally, 2022 has been a big year for KLAS development, even if it has sometimes seemed quiet from the user's side, as we made big strides in some big projects. Here's some of the highlights:
- Scribes can now unlock NLS Cartridges making it easier to repurpose physical collections and quicker to start using new white cartridges.
- To meet PNDB funding requirements, a major integration project with Rolka-Loube was implemented and is in Live use now as agencies complete their year-end reporting.
- The New WebOPAC, while not yet ready for release, is coming along beautifully. Thank you everyone for your feedback and feature requests!
- APH Integration for our IRC customers is another ongoing project which, while not yet in Live release, should be ready to go very soon.
And of course, there was much, much more--all of which can be found in the 7.7 Release Lists.
-
Farewell Message from John O.

I want to take some time to say Merry Christmas and Happy New Years to you all, my customers and friends. In my twenty years at Keystone I've tried to develop and foster relationships with all that have reached out to me for help. Keystone allowed and encouraged this and I'm thankful to them for trusting me with your care.
It's now time for me to start the next phase of my life. My wife Laura retired almost three years ago and it's time for me to join her. Friday December 30, 2022 will be my last day at Keystone. We plan to travel and enjoy spur of the moment adventures with family and friends. Plus a few “Honey Do's” that she's been listing over the years.
Thank you all and God Bless each and every one.
John Owen -
Happy Holidays from Keystone Systems!

It's been a while since we've had an "around the office" post here, so we wanted to vicariously invite all of you working this Christmas Eve (or catching up on KLASusers afterwards) to our annual holiday celebrations.
Whatever you celebrate: may your days be joyous and bright!

For the office potluck, we had an excellent array of holiday dishes, usually classics but often with a little something new. An office favorite is the soup that John Owen brought every year--that recipe was handed down to George who dutifully carries on the tradition.



After lunch, we have a white elephant gift exchange, with each person either opening a gift or stealing someone else's. There's always a lot of laughter, and just a little chaos.
On another night, we all go out for a more formal dinner, with families invited.

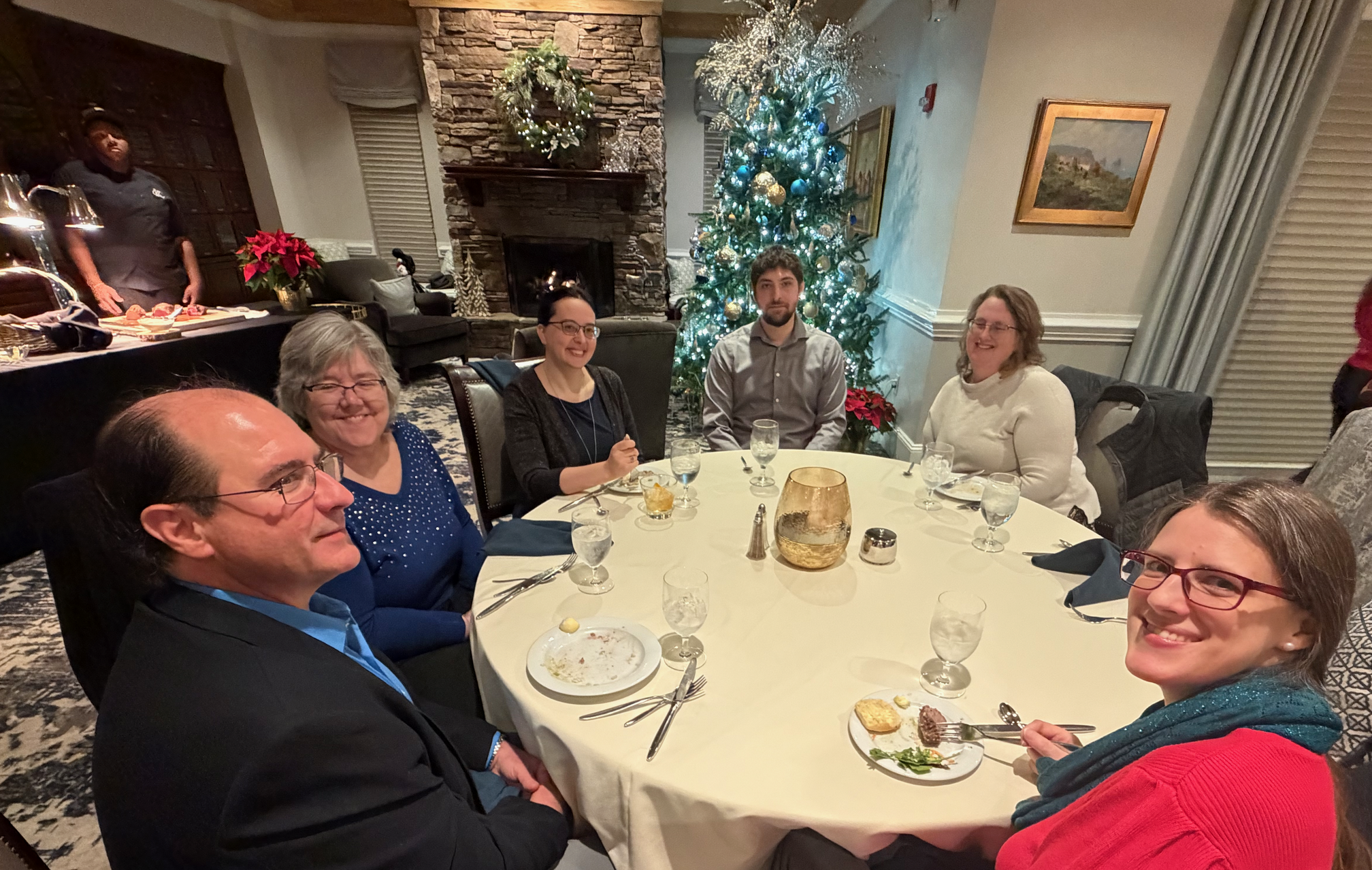


Not pictured: Drea, because she was the one making the rounds with the camera.

Dave brought a present for us all this year: new signage to try to keep the other tenants of the building out of our parking spaces!
I hope you enjoyed this glimpse of the festivities! You all are part of the Keystone family too, and we hope for a chance to see you in the next year's webinars and mini-conference--or even better, in-person at KLASusers 2027!
-
Holiday Cheer at the Keystone Office

Yesterday, we had our first Holiday office party since 2019. It was wonderful to get everyone back together, share some delicious food, and exchange some gifts. But we also did something totally new and unique: George, who joined Keystone as a developer earlier this year, brought in some liquid nitrogen, and led us in a science experiment!
We started off with a demonstration of the super-cold liquid's properties, with Katharina(our newest Customer Support Specialist) submerging a rubber ball, freezing it to the point that its once-flexible molecules were too densely packed to bounce back. Instead, when she dropped the ball, it broke apart with a loud crack!
[Video description: George dons heavy-duty protective gloves, and picks up a bottle of liquid nitrogen as he explains its properties. The Keystone staff, mostly dressed in festive holiday outfits, are gathered in a circle to watch. The nitrogen steams and boils as soon as it hits the bowl. George helps Katharina gear up in the gloves and safety goggles, then gives her a rubber bouncy ball to hold with long metal tongs. Katharina carefully dunks the ball in the nitrogen, holding it under as the liquid boils around it. Once the boiling subsides, she pulls it out, holds it straight in front of her, and drops it on a metal plate. On impact, the ball splits into three even chunks.]
Once the ball returned to room temperature, the pieces were once again soft and squishy. But the best part of the experiment was up next: ICE CREAM!!!
As the liquid nitrogen was poured into the much warmer bowl of milk and sugar, the ingredients were rapidly chilled, and the nitrogen boiled off, keeping everything light and fluffy. Within minutes, we had delicious, freshly-made soft serve!
[Video description: a long table holds two bowls with a chocolatey liquid in them. Katy and Mitake take turns stirring one bowl, while James and John C. work on the other. They are all wearing rubber gloves and safety glasses. While explaining what to do, George helps each pair get set up with a folded napkin to hold the metal bowl with, since those bowls are about to get very cold. The nitrogen is stored in tall thermoses, just like you might use for coffee or soup. More chocolate is added, and once the ingredients are ready, James and Mitake start pouring in the nitrogen. So much white fog steams up, that you can no longer see the other contents of the bowls, and James, Mitake, and George have to keep fanning the bowls for Katy and John to see what they're stirring. Gradually, the liquid mix in the bowls thickens and ices up into soft serve.]
While we can't invite all of you to the office for dessert, we hope that you all will have the chance to share some holiday joy and wonder of your own--whether it comes in the form of a science experiment, a gift exchange, or just a chance to catch up with friends and family.
From all of us to all of you, Happy Holidays!
[Video description: a collage of close-up photos of the ice cream making process surround a video clip from another angle. The photos show the table with everyone preparing to make the ice cream, the Keystone staff gathered around filming or watching, bowls being held tight and stirred just as the ingredients start to form up, and finally a bowl of delicious-looking chocolate ice cream.]
-
John Owen's Retirement Celebration

After over 20 years of service in Keystone's Customer Support department, John Owen retired on December 30, 2022. Last Thursday, Keystone staff gathered at Margaux's Restaurant in Raleigh, NC to enjoy a celebratory dinner in honor of John and his dedication to his clients and coworkers. John will be remembered for his calming voice, adeptness at diagnosing and fixing various types of mailing card printers, and his unrivaled selection of shoes. While his presence is already missed, we're excited to have had Katharina training for six months in anticipation of his departure.
John, we wish you all the best in the next chapter of your life! You and Laura will always be a part of the Keystone family!
Below are a selection of photos taken at John's retirement celebration:




-
The Stars of Keystone's Staff - George

Our blog post this week is the latest in our "Stars of Keystone" series. This time we're highlighting our newest Keystone staff member, so let's welcome and learn more about George!
Basic Stats:
Name of Staff Member: George Martell
Year Hired: 2022
Current Job Title: Software Development Specialist
Getting to Know You Q&A:
Q: What are you most excited about / looking forward as a new KLAS developer?
A: Much of my life has been spent serving various communities and trying to develop tools that enable people to do more. I love to pursue the promise of technology where it allows us to do things that we couldn’t do before, do them more efficiently, or do things in new ways that were previously thought impossible. Technology has the power to transform lives, and being able to contribute my small part to that gives me purpose.
Q: What did you do before working for Keystone?
A: IT, Safety, and Security at NC State University. Taught Nuclear Power, Radiological Chemistry, electronics repair and design at a Department of Energy Site. Operated, Maintained, and Repaired Nuclear reactors for 10 year, 5 of which I lived on a submarine.
Q: What are your hobbies outside of work?
A: plants, backpacking, pretty much anything outdoors, building things.
Q: If you could go anywhere on vacation, where would you go?
A: Somewhere I haven’t been before, I love to explore.
Q: Do you have any pets? If so, what kind and what are their names?
A: Turtle, had her since I was in elementary school, her name is Urtle (give me a break on the name, I was in elementary school).

A map of places where George has traveled.
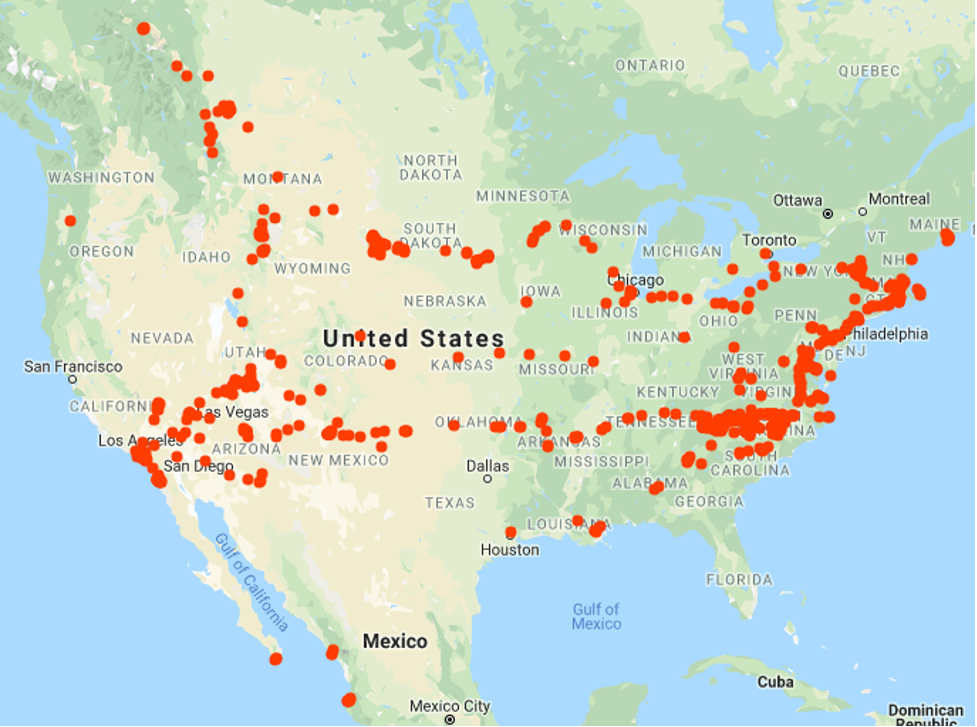
-
The Stars of Keystone's Staff - Katharina

In this week's blog post and latest in our "Stars of Keystone" series, we're excited to introduce the newest member of the Keystone Customer Support Team! She's been with us since early July, and we are already benefiting from her contributions to our support services. So, please join us in welcoming Katharina!
Basic Stats:
Name of Staff Member: Katharina Stevens
Year Hired: 2022
Current Job Title: Customer Support Specialist
Getting to Know You Q&A:
Q: What are you most excited about / looking forward to as a new KLAS customer support specialist?
A: I think reading and access to books is very important. In America we are blessed with an over abundance of resources though we don’t always realize it. I’m excited to be a small part in promoting and improving access to reading materials.
Q: What did you do before working for Keystone?
A: I was the school librarian at an international school in East Africa for nine years. The only wildlife I saw in my yard were geckos, birds and stray cats but coworkers had monkeys that would visit.
Q: What are your hobbies outside of work?
A: Reading, traveling, board games and being an aunt.

Katharina standing in front of Victoria Falls, Zimbabwe.Q: If you could go anywhere on vacation, where would you go?
A: New Zealand & Australia and then somewhere in South America so I can say I’ve been to all continents (except Antarctica).
Q: Do you have any pets? If so, what kind and what are their names?
A: While in East Africa my housemate and I found two puppies in the field across the street. The mama was the neighborhood stray so we ended up taking the puppies. Roo and Stoney (named after a Ugandan ginger soda) needed a bath every day for a month to get them clean and bug free.


-
Wishing you a hearty & healthy Thanksgiving

Keystone Systems' office will be closed Thursday, November 24 and Friday, November 25 in observance of Thanksgiving. But before we send out staff off to gather with friends and family, we asked them to share some of their favorite holiday recipes.
We're all grateful that you're part of our KLAS users family, and hope you all have a lovely holiday!
From Nancy Honeycutt, Customer Support Manager:
Mom would always make refrigerator rolls. One time she left a cookie sheet with rolls rising on the counter while we went to pay a Christmas visit to neighbors. When we came back, the cookie sheet was on the floor, completely cleaned off, and the dog was suspiciously innocent (and sick later that night).
Refrigerator Rolls
Ingredients
- 1 yeast cake
- 1/2 cup lukewarm water
- 2/3 cup shortening
- 1 tsp salt
- 1/2 cup sugar
- 1 cup mashed potatoes
- 1 c scalded milk
- 6-8 cups flour
- 2 eggs
Instructions
- Mash potatoes.
- Add shortening, sugar, salt & eggs. Cream well.
- Dissolve yeast in water, add to lukewarm milk. Then add to potato mixture.
- Add sifted flour to make stiff dough. Knead lightly.
- Place in casserole and brush top with butter.
- Cover tightly and place in refrigerator until ready to use.
- About 1/2 hour before baking time remove and shape in rolls.
- Cover and let rise until light.
- Cook at 400* for 10-12 minutes.

From Marion Campbell, Customer Support Specialist:
One of my families favorite food for the holidays are sweet potato biscuits. One holiday when my nephews were 9,10,10-ish, the biscuits came out early and were warming on the table. Slowly, they each snuck a biscuit or 3... when it was time to eat there were no biscuits left in the basket because the boys had eaten them all. Needless to say, a 2nd tray came out soon after but the boys were full from biscuits and did not eat much else that year. They all had tummy aches from the biscuits and now the rule of biscuits is: only eat 2, more than 2 and you will not be happy.
Sweet Potato Biscuits
Here's what's cookin': Sweet Potatoe Biscuits
From the kitchen of: Dorothy WrightIngredients
- 4 cups packed of sweet potatoes
- 1 cup Crisco
- 1 cup of sugar
- 4 cups of self-rising flour
Instructions
- Peel sweet potatoes and boil until done.
- While hot mash and add to them Crisco, sugar, and flour.
- Mix good.
- Work into biscuits.
- More flour is sometimes needed. Depends on wetness of potatoe to be able to handle the mixture.
- Bake at 350* for 20-30 minutes. Depending on size of biscuits.
- Mix handles better if cooled.
- Bake on greased cookie sheet.

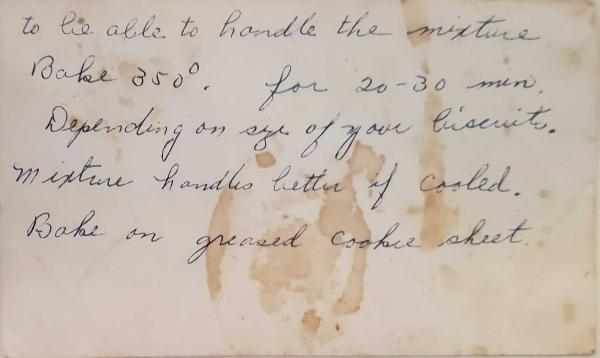
From Katharina Stevens, Customer Support Specialist:
Among many other things I am thankful for are ovens that allow you to set a specific temperature and that cook evenly. Baking in East Africa was always an adventure. One summer I brought the ingredients for pumpkin pie back with me to make at Thanksgiving. It didn't turn out very pretty. Had to cut off the burnt parts, and take a moto taxi across town to buy some cinnamon whipped cream to hide the mess. Tasted good though.
Seriously though, I'm immensely thankful for family, friends and the blessing that is America. We've come a long way from the 5 kernels of corn that that Pilgrims had to eat each day that first winter of 1620.


From Drea Callicutt, Director of Marketing, Sales & Communications:
One of our favorite holiday dishes is more recent. My sister learned to make duck fat roasted potatoes while she was in graduate school in Edinburgh. She made them for us for the first time for Christmas dinner when were visiting her in Leeds, and they’re now a staple whenever she’s joins us for a holiday meal.
This recipe from Vindulge.com is pretty much how she makes hers:
Roasted Duck Fat Potatoes
Equipment
- Large Sheet Tray
- Parchment Paper
- Ingredients
- 2 pounds yellow potatoes, cut into ½ inch dice
- 2 tablespoons salt (for boiling the potatoes)
- ¼ cup duck fat
- 1 teaspoon kosher salt
- 1/2 teaspoon pepper
- 1 tablespoon freshly graded parmesan
- 1 teaspoon thyme, finely diced
Instructions
- Preheat the oven to 450 degrees Fahrenheit.
- In a large pan, place the diced potatoes and fill with cold water until it just covers the potatoes. Add 2 tablespoons of salt in the water.
- Bring to boil. Start timer for 14 minutes. When the potatoes come to boil, reduce heat to a simmer. After timer runs out, strain the potatoes in a colander.
- In a large bowl, place the potatoes and then add the duck fat, additional kosher salt, and pepper. Stir with your hands to incorporate and then place on a large baking sheet lined with parchment paper.
- Place in the oven, and toss the potatoes every 15 minutes. The duck fat will slowly brown the potatoes, especially the sides touching the pan. So turning the potatoes every 15 minutes will help get more of the crunch on the surface area of the potatoes.
- After 40-45 minutes, the potatoes should be golden in color. Remove and place them in your favorite serving dish. Top with the parmesan and thyme. Serve warm.

